New treatment may transform care for rare type of hydrocephalus
Older adults with a brain disorder called normal pressure hydrocephalus (NPH) could one day have access to a minimally invasive treatment under review by the country's leading neurosurgery programs.
Henry Ford Health is among a select handful of institutions across the U.S. participating in a clinical trial to evaluate a potentially breakthrough approach in treating NPH, a condition that occurs when excess cerebrospinal fluid builds up in the brain, putting pressure on surrounding tissue.
"Brain surgery to divert fluid from the brain into the abdomen has long been the standard treatment for NPH, but not every patient is a good candidate for such a complex procedure," said Henry Ford Health neurosurgeon Dr. Pouya Entezami, who enrolled his first patient in the clinical trial this spring. "A safe, less invasive treatment could transform care for this debilitating and often underdiagnosed condition."

NPH affects more than 800,000 Americans, mostly those over age 60. Symptoms include difficulty walking, urinary incontinence and cognitive impairment that often resembles dementia.
Legendary musician Billy Joel’s recent disclosure that he suffers from NPH has raised awareness of the condition, which is frequently misdiagnosed as Alzheimer's disease, Parkinson's disease or general aging.
For decades, treatment has involved surgeons drilling a hole in the skull and inserting a ventriculoperitoneal (VP) shunt to drain excess fluid from the brain into the abdomen. However, recovery time and potential complications can make surgery risky for some patients who are elderly, frail or have other underlying medical conditions.
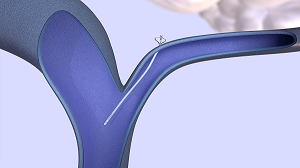
A new endovascular shunt developed by CereVasc Inc., a clinical-stage medical device company, could offer a less invasive alternative. The company's eShunt ® System is implanted near the base of the skull using small catheters, allowing the fluid to be reabsorbed into the bloodstream. The shunt is placed using access through a small incision in the leg during a procedure that takes about an hour.
As part of its bid for FDA approval, CereVasc has launched a randomized, controlled trial investigating the safety and effectiveness of the device. The STRIDE clinical trial will enroll 200 patients with NPH, randomly assigning them to receive either the traditional VP shunt or the new eShunt. Each participant will be followed for at least one year to assess outcomes.
Henry Ford Health is the only site in Michigan participating in the study, which also includes neurosurgery programs at Yale Medicine and VCU Health.
###
MEDIA INQUIRIES: mediarelations@hfhs.org
.svg?iar=0&hash=F6049510E33E4E6D8196C26CCC0A64A4)

/hfh-logo-main--white.svg?iar=0&hash=ED491CBFADFB7670FAE94559C98D7798)